Today almost no credible evidence suggests that cannabis belongs on Schedule I of the Controlled Substances Act, alongside drugs like heroin. This position has stifled medical research of the drug and its component chemicals for 39 years, making research extremely expensive and arbitrarily difficult to secure compared to that of much more harmful drugs.
A few organizations have spent enormous amounts of money going through the formal petition process in good faith to reschedule cannabis, and each petition has been met with blatant obstructionism; lengthy delays, arbitrary dismissals, last-minute over-rulings. The current petition hasn’t been acted on since its submission seven and a half years ago.
Two signs suggest we may soon see a relaxation of the unjustified limitations imposed on researchers.
1. One of the last influential and independent voices claiming that the drug had no medicinal properties (ignoring an absurd amount of evidence) was the American Medical Association. Today they finally fixed that. They make a very reasonable request:
Our American Medical Association (AMA) urges that marijuana’s status as a federal Schedule I controlled substance be reviewed with the goal of facilitating the conduct of clinical research and development of cannabinoid-based medicines. This should not be viewed as an endorsement of state-based medical cannabis programs, the legalization of marijuana, or that scientific evidence on the therapeutic use of cannabis meets the current standards for a prescription drug product. [AMA statement pdf]
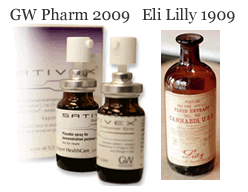 2. Sativex is an oral spray pharmaceutical made from the whole cannabis plant; it should look suspiciously familiar to these medicinal extracts used around the turn of the century. Already approved in Canada, it’s breezing through U.S. phase III clinical trials for M.S. treatment, with FDA approval potentially a couple years away (barring political shenanigans). Upon approval, whole-plant cannabinoids–not just the isolated THC in dronabinol–can finally be easily studied for the treatment of other ailments.
2. Sativex is an oral spray pharmaceutical made from the whole cannabis plant; it should look suspiciously familiar to these medicinal extracts used around the turn of the century. Already approved in Canada, it’s breezing through U.S. phase III clinical trials for M.S. treatment, with FDA approval potentially a couple years away (barring political shenanigans). Upon approval, whole-plant cannabinoids–not just the isolated THC in dronabinol–can finally be easily studied for the treatment of other ailments.
If a cannabis extract proves to be as effective and safe as many scientists expect, I think it’s likely the public will demand more research in this area via some alteration of the Controlled Substances Act. My bet is that politics will keep marijuana in Schedule I, and (a generic name for) Sativex will be placed in Schedule III. This would ease research considerably while ensuring GW Pharmaceuticals enjoyed a healthy monopoly for years to come.
As for why cannabinoid research is so damned important, see GW Pharm’s site.
generic name for Sativex is nabiximols —
if they schedule that in 3 and the plant that it derives from (it’s a liquid co2 extract after all), I think that would be absurd and intolerable. Can’t call cannabis inherently dangerous if a liquid form can be Rx’ed over the phone.